Select Device Freestyle Lingo Freestyle Libre 3 Freestyle Libre 2 Freestyle Libre 14 Day Dexcom G7 Dexcom G6 Dexcom Stelo Guardian Connect Medtronic Simplera Eversense Meter Omnipod Omnipod Dash Medtronic 780G Medtronic 630G Tandem t:slim X2 Bigfoot Unity Medtronic InPen Tempo Pen NovoPen 6/NovoPen Echo Insulin Pen Afrezza CeQur Simplicity Patch V-Go Patch Omnipod GO Patch Syringe and Vial Beta Bionics iLet Pancreas Control IQ with Dexcom Control IQ with Freestyle Libre Medtronic 780G with Smart Guard Omnipod 5 Tandem Mobi Tidepool Loop twiist DIY Loop Apple Series 6/7/8/9/10/SE (2nd Generation) Garmin vívosmart 5™ Garmin Venu Sq 2 Garmin Venu 3 Garmin vivoactive 5 Garmin Fenix 8 Garmin vivofit jr 3 Garmin Forerunner 165/265/965 Garmin epix (Gen 2) Garmin Enduro 3 Fitbit Ace LTE Fitbit Charge 6 Fitbit Inspire 3 Fitbit Versa/Versa 2/4/Versa Lite Fitbit Sense 2 Fitbit Luxe Google Pixel 2/3 Samsung Galaxy 4/5 Xiaomi Mi Band 9 Oura Ring Whoop 4.0 Withings ScanWatch 2 Withings ScanWatch Lite Pedometer 3D Pedometer
Select Device Freestyle Lingo Freestyle Libre 3 Freestyle Libre 2 Freestyle Libre 14 Day Dexcom G7 Dexcom G6 Dexcom Stelo Guardian Connect Medtronic Simplera Eversense Meter Omnipod Omnipod Dash Medtronic 780G Medtronic 630G Tandem t:slim X2 Bigfoot Unity Medtronic InPen Tempo Pen NovoPen 6/NovoPen Echo Insulin Pen Afrezza CeQur Simplicity Patch V-Go Patch Omnipod GO Patch Syringe and Vial Beta Bionics iLet Pancreas Control IQ with Dexcom Control IQ with Freestyle Libre Medtronic 780G with Smart Guard Omnipod 5 Tandem Mobi Tidepool Loop twiist DIY Loop Apple Series 6/7/8/9/10/SE (2nd Generation) Garmin vívosmart 5™ Garmin Venu Sq 2 Garmin Venu 3 Garmin vivoactive 5 Garmin Fenix 8 Garmin vivofit jr 3 Garmin Forerunner 165/265/965 Garmin epix (Gen 2) Garmin Enduro 3 Fitbit Ace LTE Fitbit Charge 6 Fitbit Inspire 3 Fitbit Versa/Versa 2/4/Versa Lite Fitbit Sense 2 Fitbit Luxe Google Pixel 2/3 Samsung Galaxy 4/5 Xiaomi Mi Band 9 Oura Ring Whoop 4.0 Withings ScanWatch 2 Withings ScanWatch Lite Pedometer 3D Pedometer

Summary
The twiist AID system was cleared by the FDA for use with CGMs that have received FDA designation as integrated continuous glucose monitors (iCGMs). As the twiist AID system gets closer to commercial availability, Sequel Med Tech will publicize the list of compatible CGM
*Recently FDA approved for people with type 1 diabetes ages 6 and up.
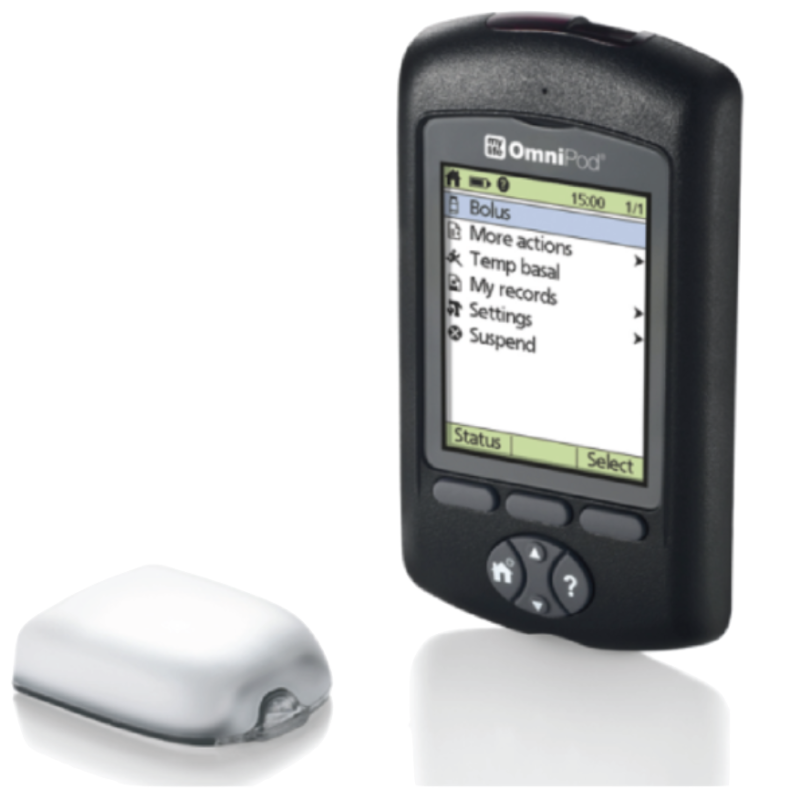
The OmniPod is the only tubeless pump option. A Personal Diabetes Manager (PDM) is used to give insulin doses to the pod, which remains attached to the body. This pump is one of few that is completely waterproof.
Components

twiist : Pump
Pump can be worn with a pump clip, adhesive patch or in a pocket.
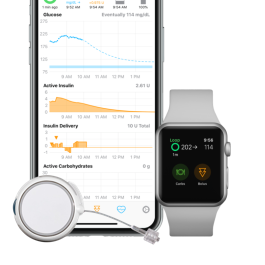
twiist : Controller
Pump is controlled by the twiist app on an iPhone. Data from both the pump and CGM are viewable on the twiist app Home Screen.

Twiist : Infusion Set
An infusion set contains the thin plastic tubing that delivers insulin from the pump to the body.

Omnipod : Pump
Pods come with reservoirs and directions for application. They can be applied anywhere on the body that is comfortable.
Omnipod : Reservoir
Fill reservoir is enclosed with the Pod and used to manually fill the Pod with insulin.
Omnipod : Controller
Personal Diabetes Manager (PDM) is used to give insulin doses to the pod.
Device Details
Overview
This system automatically adjusts insulin delivery according to continuous glucose monitor (CGM) readings and predicted blood sugar levels. It is the first FDA-approved AID system with Apple Watch compatibility.
This is a discreet pump option without tubing but will require a controller to dose.
Affordability and Access
$$$$
No insurance coverage data. Anticipated to be provided through Pharmacy.
$$$$
Widely covered for insulin-dependent type 1 and 2. Pump can be provided by pharmacy or DME. Price range; 0-$200/month.
Data Monitoring Options
Yes
Compatible with Tidepool - a web-based diabetes data management system.
Yes
Pump data can be accessed by clinicians on Diasend by Glooko.
Data View Options
Yes
Data is stored and can be viewed directly from the twiist app on the mobile device (iPhone), and can be linked to an Apple Watch for viewing data as well.
No Options
Duration and Storage
3-day pump 10-day sensor
Sensors last 10 days, and transmitters last 90 and work at 50ºF – 108ºF. The reservoir holds 300 units and is disposable, and the pump can be used for up to 3 years.
3 Days
Pod can be worn for up to 72 hours and work from 41ºF – 104ºF.
Vision / Auditory / Dexterity
YES text to speech
YES vibration alerts
A LOT of dexterity needed
NO text to speech
NO vibration alerts
A LOT of dexterity needed
Patient Considerations
Active Lifestyle
Waterproof. The pump, when connected to the cassette, has a rating of IP28, indicating protection from continuous immersion in water. The pump can tolerate immersion to depths of up to 12 feet (3.7 m) for 1 hour.
Waterproof. Wear the Pod in the shower, in the pool, or in the ocean. The PDM is not waterproof and must be charged daily.
Avoiding Highs and Lows
Automatically adjusts insulin delivery. Increases or decreases insulin delivery to meet glucose targets. Insulin delivery settings can be aggressive or conservative.
Personalized dosing. Pods can deliver personalized doses of rapid-acting insulin based on the rates programmed into the Personal Diabetes Manager (PDM) device. The ability to set zero basal rates is not available.
Comfort
Low-profile. Low profile with an adhesive design that prevents the pump from catching on other objects.
Free of tubing. Pods do not have tubes like traditional insulin pumps, allowing for more freedom of movement.
Easy Insulin Dosing
Customizable. Can bolus from phone, smartwatch, or directly from the device without needing to be attached to your phone. The device also directly measures the volume (amount) and flow (speed) of insulin delivered with every micro-dose
Customizable dosing. Basal and Bolus in 0.05 increments. No interruption in insulin delivery (pods stay on during bathing and swimming).
Easy to Use
Watch compatible. The twiist AID system is the first FDA-approved AID system that can be directly controlled from an Apple Watch.
Fewer parts. The PDM has button navigation. Pod has fewer parts than tubed pumps.
Fewer Fingersticks
Factory-calibrated sensor. Fingersticks needed for backup only.
Frequent finger-sticks are required.
Privacy
Discreet. The pump is in two pieces - the top (algorithm and battery) and the bottom (insulin chamber and AVS technology)
Somewhat discreet. The pod attaches directly to the body with no tubing, but the pod is bigger than an infusion set. Pod does not have vibrate option.